The molecular mechanisms of rice SPX2– PHR2 complex sensing phosphate signals and regulating phosphate homeostasis were revealed by the National Key Laboratory of Crop Genetic Improvement, Hubei Hongshan Laboratory, the Center for Protein Research from the College of Life Science and Technology, HZAU. The journal Nature Communications published their latest findings “Mechanistic insights into the regulation of plant phosphate homeostasis by the rice SPX2 – PHR2 complex”.
Phosphorus is an essential nutrient limiting plant growth, development, and crop productivity. But excessive use of phosphate (Pi) to increase agricultural production has triggered environmental problems. Therefore, it is of great theoretical and practical significance to study the maintenance mechanism of plant phosphate homeostasis. Previous studies have shown that under Pi starvation, rice (Oryza sativa) PHR2 (OsPHR2) binds to a cis-element (P1BS) in the promoter of various PSI genes and up-regulates their transcription, thus optimizing rice Pi acquisition and utilization. In contrast, under Pi sufficient conditions, SPX2 receptor protein senses the Pi-level correlated PP-InsPs signal, then binds to OsPHR2 and inactivates its transcriptional activity, maintaining the Pi homeostasis and avoiding Pi poisoning. But how the SPX2 senses and transduces the Pi-level remains largely unknown.
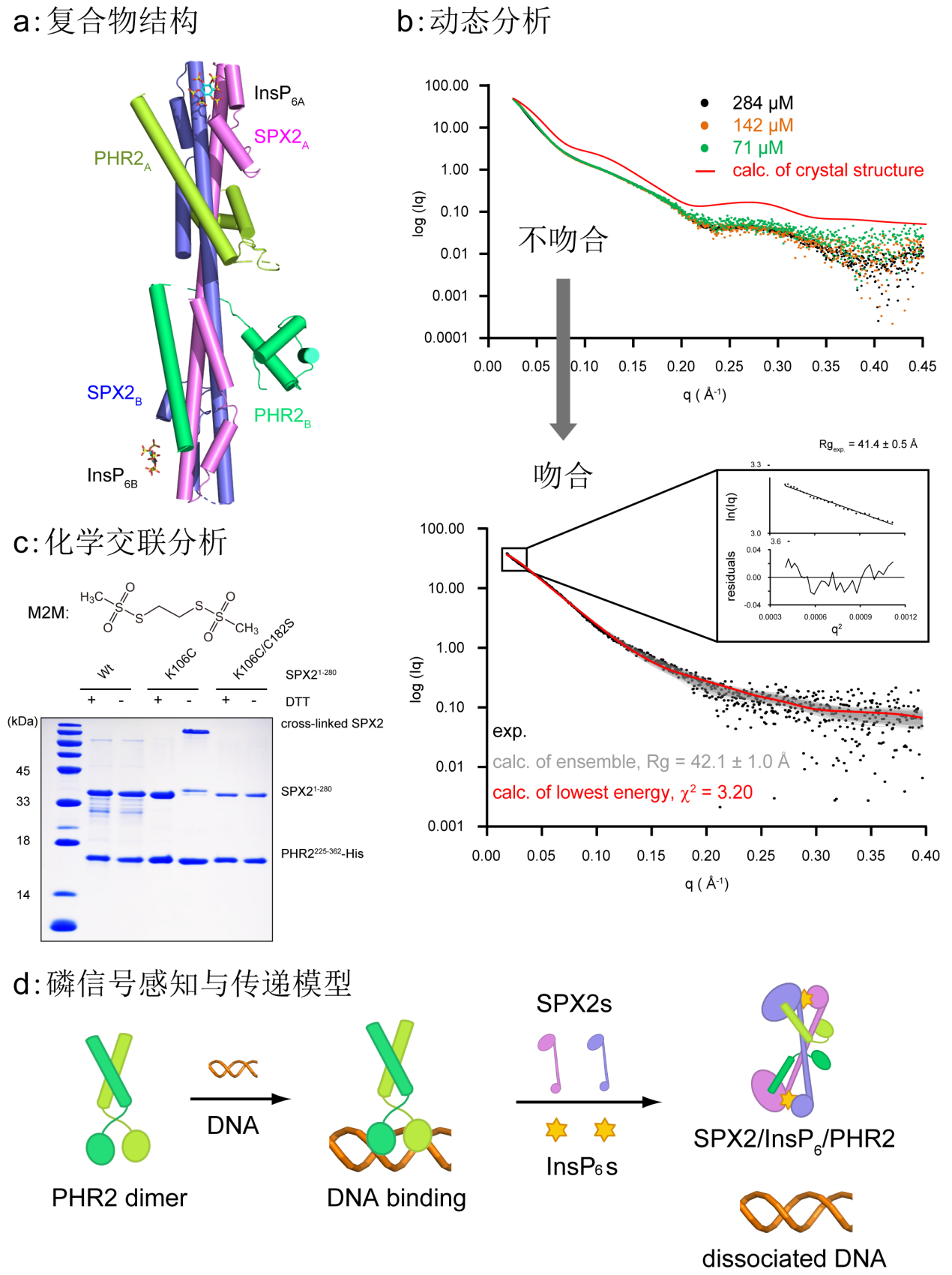
Fig. 1 Overall structure of the ternary complex, dynamic analysis and chemical crosslinking analysis were applied to elucidate the molecular mechanisms of SPX2 receptor to sense and transduce Pi signals, thereby attenuating transcriptional activity of PHR2.
The team revealed the mechanism of SPX2 receptor sensing and transducing the inositol phosphate (InsP6) to regulate rice Pi homeostasis at molecular level by integrating structural biology strategies, various biophysical analysis, structural comparison and dynamic analysis (Fig. 1). SPX2 perceives InsP6 with unique domain-swapped conformation through the dynamic changes of oligomeric states. The signalling-active SPX2 dimer binds two copies of PHR2, and each polypeptide of the SPX2 dimer binds to the PHR2 MYB domain and PHR2 CC domain, respectively. This unique binding model spatially excludes PHR2 from binding to PIBS, and by disrupting the dimerization of PHR2, thereby attenuating its transcriptional activity and maintaining Pi homeostasis. This study mechanistically rationalizes the molecular basis of rice Pi dynamic balance, which is significant to genetic improvement and sustainable fertilization of the agronomic traits. Also, it paves the way for the selection and breeding of rice varieties with different Pi acquisition capacity according to local conditions.
Dr. Guan Zeyuan , Dr. Zhang Qunxia and Zhang Zhifei from the College of Life Science and Technology, HZAU are the co-first authors of this paper, and Prof. Liu Zhu is the corresponding author. The Center for Protein Research, HZAU provided facilities support and the NCPSS at Shanghai Synchrotron Radiation Facility assisted in data collection.The research was supported by the Ministry of Science and Technology, the National Natural Science Foundation of China (32071226 to Z.L.), and the Fundamental Research Funds for the Central Universities (Program No. 2662019PY004 to Z.L.). Dr. Guan also acknowledges the support of National Postdoctoral Program for Innovative Talents (BX2021108), China Postdoctoral Science Foundation and Baichuan Postdoctoral Program of the College of Life Science and Technology, HZAU.
Source: http://news.hzau.edu.cn/2022/0324/62806.shtml
Translated by: Liu Mengyan
Supervised by: Jin Bei