Recently, PNAS (Proceeding of the National Academy of Sciences of the United State of America) has published a paper online titled “Seneca Valley virus attachment and uncoating mediated by its receptor anthrax toxin receptor 1” . The findings are jointly discovered by our animal bathogenic microorganisms team led by Pro. Qian Ping and the research team of Tsinghua University led by Pro. Lou Zhiyong. The paper for the first time revealed the mechanism of Seneca Valley virus's attachment and uncoating mediating by ANTXR1 from the aspect of structural biology. Previously, Qian Ping's team has already published two related research articles in Virology Journal: the isolation and full-genome sequencing of Seneca Valley virus in piglets from China in 2016 and Seneca Valley Virus Suppresses Host Type I Interferon Production by Targeting Adaptor Proteins MAVS, TRIF, and TANK for Cleavage in 2017.
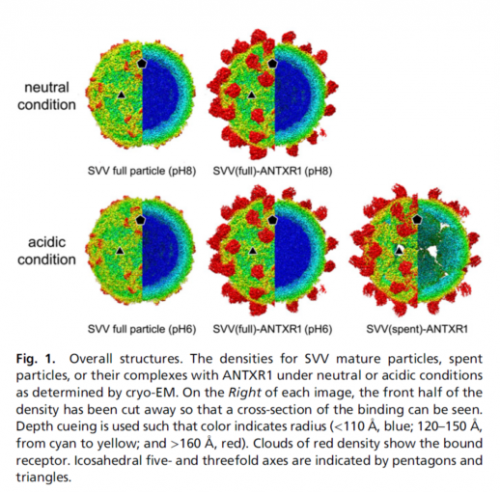
Seneca Valley virus (SVV), a prototypic member of the Senecavirus genus within the Picornaviridae family, is characterized by oncolysis and can selectively infect the neuroendocrine cancers. It has shown promise as a cancer therapeutic in preclinical studies and early-phase clinical trials. As the receptor for SVV, anthrax toxin receptor 1 (ANTXR1) is necessary for SVV to invade into host cells.
The joint research team determined atomic structures of mature SVV particles alone and in complex with ANTXR1 in both neutral and acidic conditions and the atomic structures for SVV–ANTXR1 in different conditions by using cryoelectron microscopy and 3D reconstruction technology. Furthermore, they discovered that the engagement of SVV was significantly different from other reported picornavirus–receptor complexes. SVV engages ANTXR1 mainly by the joint participation of VP2 DF and VP1 CD loops. Assisted by the VP1 CD loop, the SVV VP2 DF loop plays an essential role in binding ANTXR1.
In addition, one of the most significant findings is that a serine-to-alanine mutation at VP2 177 position on the interface largely increases proliferation. The molecular details for SVV–ANTXR1 attachment and uncoating shown in this work provide clues to optimize SVV as an oncolytic reagent with higher efficacy and lower immunogenicity.
This paper’s co-first authors are Liu tingting, a PhD candidate from College of Animal Sciences & Technology of HZAU and three PhD candidates from Tsinghua University. Prof. Qian Ping and Prof. Lou Zhiyong are co-corresponding authors. This work was supported by Fundamental Research Funds for the Central Universities Grant and National Program on Key Research Projects of China Grants.
Link:https://doi.org/10.1073/pnas.1814309115
Translated by:Yin Ruixuan
Supervised by:Guo Haiyan